Buy Elevidys(delandistrogene moxeparvovec-rokl) | 100% best sale
$2,900,000.00
Elevidys (delandistrogene moxeparvovec) is a gene therapy treatment for Duchenne muscular dystrophy (DMD)
Description
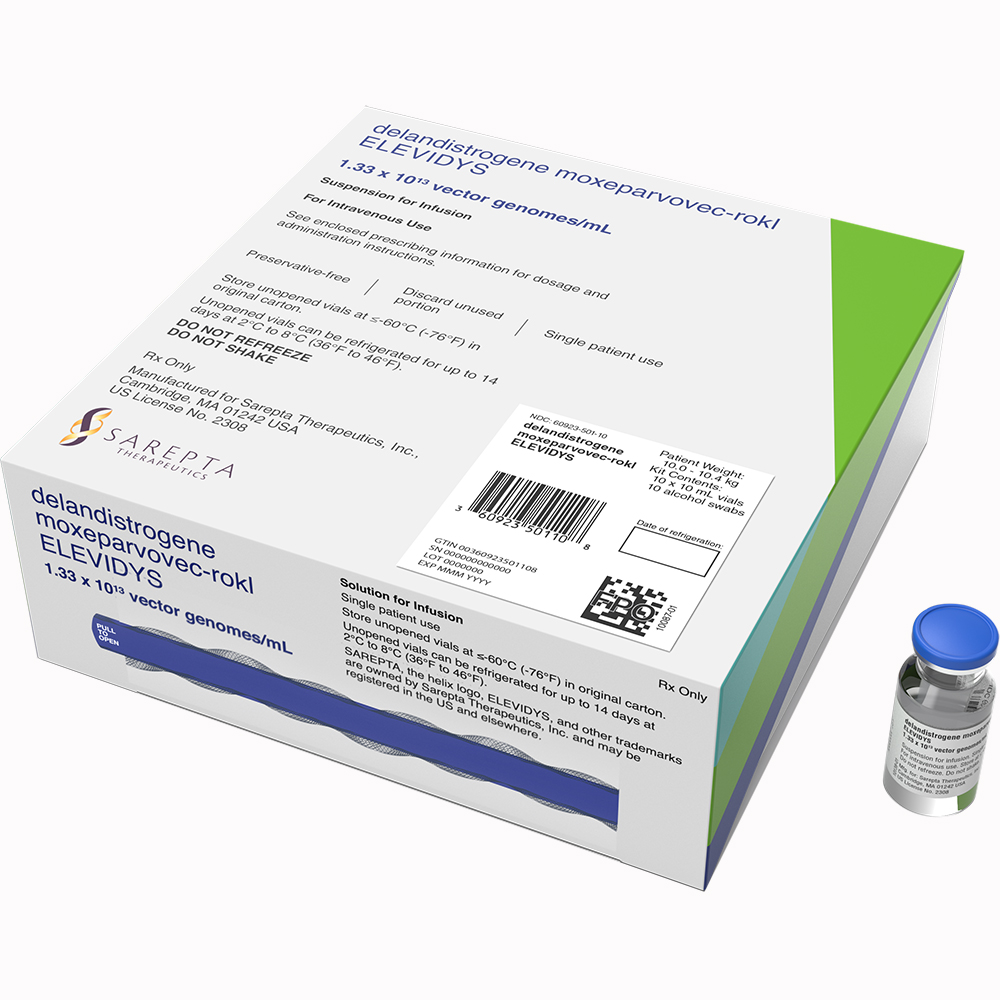
What is Elevidys(delandistrogene moxeparvovec-rokl)
ELEVIDYS is a prescription gene therapy used to treat ambulatory and non-ambulatory people with Duchenne muscular dystrophy who are at least 4 years old and have a confirmed mutation in the dystrophin gene.
Use in non-ambulatory people is approved under accelerated approval, which allows for drugs to be approved based on a marker that is considered reasonably likely to predict a clinical benefit. Treatment with ELEVIDYS increased the marker, ELEVIDYS micro-dystrophin (also called “micro-dystrophin”). Verification of a clinical benefit may be needed for ELEVIDYS to continue to be approved for non-ambulatory people with Duchenne.
What is Elevidys used to treat?
Elevidys is a one-dose treatment. After a single infusion, the drug is thought to work long-term to treat DMD in certain people. How long does Elevidys take to work? Elevidys begins to work shortly after you receive the infusion.
How many doses of Elevidys?
Who can get Elevidys?
What is Duchenne Muscular Dystrophy?
Duchenne muscular dystrophy is a serious but rare genetic condition that becomes worse over time, leading to weakness and wasting away of the body’s muscles. The cause of Duchenne muscular dystrophy is a defective gene that codes for the dystrophin protein, an important component of muscle cells.
The lack of dystrophin causes symptoms such as trouble walking and running, falling frequently, fatigue, learning disabilities/difficulties, heart issues due to the impact on the heart muscle, and breathing problems due to weakness of respiratory muscles involved in breathing.
Symptoms typically begin in childhood, often between 3 to 6 years old, and as the disease progresses, life-threatening heart and respiratory problems can occur. Disease severity and life expectancy do vary, but patients often succumb to the disease in their 20s or 30s because of heart and/or respiratory failure.
Warnings
Infusion-related reactions, including hypersensitivity reactions and anaphylaxis, have occurred during or up to several hours following infusion. Patients are closely monitored during and for at least 3 hours after the end of infusion for signs and symptoms of infusion-related reactions, including tachycardia, tachypnea, lip swelling, difficulty breathing, nasal flaring, urticaria, flushing, lip pruritus, rash, cheilitis, vomiting, nausea, rigors, and pyrexia.
Acute serious liver injury has been observed when using delandistrogene moxeparvovec-rokl. Before using this medicine, a liver function test should be performed. Then, liver function (clinical exam, GGT, and total bilirubin) should be monitored weekly for the first three months following delandistrogene moxeparvovec-rokl infusion.
Immune-mediated myositis (an immune response affecting muscles). Patients should contact their healthcare provider immediately if they experience any unexplained increased muscle pain, tenderness, or weakness, including difficulty swallowing, difficulty breathing, or a weak voice, as these may be myositis symptoms.
Acute serious myocarditis (inflammation of the heart) and troponin-I elevations have been observed following Elevidys infusion in clinical trials. Patients should contact their healthcare provider immediately if they experience chest pain and/or shortness of breath.
Pre-existing Immunity against AAVrh74 In AAV-vector based gene therapies. Preexisting anti-AAV antibodies may impede transgene expression at desired therapeutic levels. Following treatment with this infusion, all patients developed anti-AAVrh74 antibodies. Perform baseline testing for the presence of anti-AAVrh74 total binding antibodies before infusion. Elevidys should not be administered in patients with elevated anti-AAVrh74 total binding antibody titers (≥1:400)
Infections. While taking corticosteroids, an infection before or after the Elevidys infusion could lead to more serious complications. Contact a healthcare provider immediately if symptoms suggestive of infection are observed (e.g., coughing, wheezing, sneezing, runny nose, sore throat, or fever).
How will I receive Elevidys?






Reviews
There are no reviews yet.