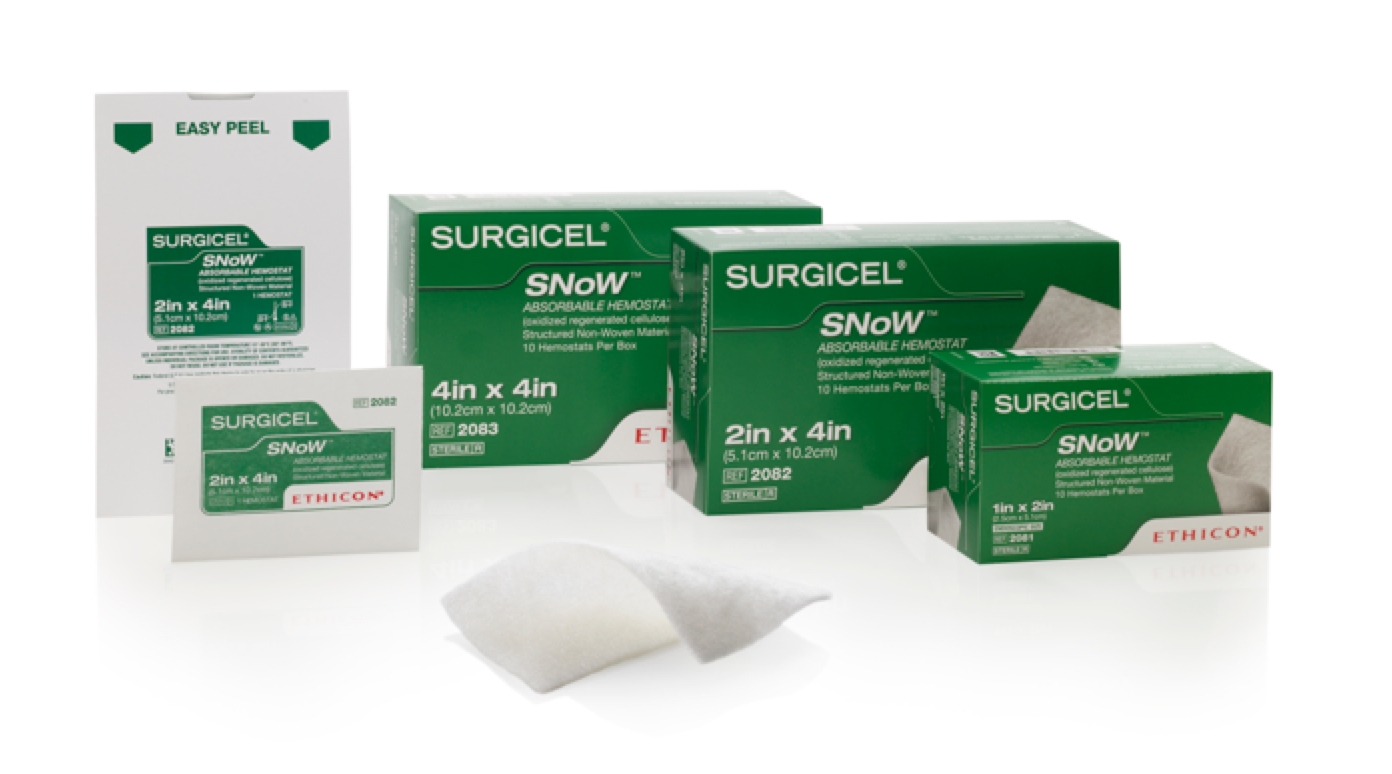
Surgicel 2082 – SNoW® Hemostat, 2″ x 4″
$605.00
Surgicel 2082 – SNoW® Hemostat, 2″ x 4″
Most products that we sell are Medical Products and are marked “RX” or “to sale by or on order of a physician”. These Products are sold to Licensed Medical Practitioners or Medical Facilities ONLY. Consumer seeking to purchase for Personal Use are required to obtain a Physician Prescription.
Description
Surgicel 2082 – SNoW® Hemostat, 2″ x 4″
-Size: 2in x 4in
-Bleeding Situation: Continuous Oozing
-Absorption Time: 7-14 days
-Storage Requirements: Store at controlled room temperature 59º-86ºF (15º-30ºC)
-Preparation Time: Ready out of package
-Shelf Life: 2 Years
-Material/Composition: Oxidized Regenerated Cellulose
More about Surgicel 2082 – SNoW® Hemostat, 2″ x 4″
Additional Specifications
Description
Structured non-woven fabric, needle punched with interlocking fibers
Brand
SURGICEL SNoW®
Size
2in x 4in
Bleeding Situation
Continuous Oozing
Hemostatic Agent & Accessory
Hemostatic Agent
Absorption Time
7-14 days
Storage Requirements
Store at controlled room temperature 59º-86ºF (15º-30ºC)
Preparation Time
Ready out of package
Shelf Life
2 Years
Material/Composition
Oxidized Regenerated Cellulose
NDC/DISTRIBUTOR CODE
63713-0020-82
Category
Adjunctive Hemostasis
QTY/BX
10
Indication
SURGICEL Absorbable Hemostat (oxidized regenerated cellulose) is used adjunctively in surgical procedures to assist in the control of capillary, venous and small arterial hemorrhage when ligation or other conventional methods of control are impractical or ineffective. SURGICEL SNoW Absorbable Hemostat can be cut to sixe for use in endoscopic procedures.
MOA
Provides a matrix for platelet adhesion and aggregation
https://www.hospeq.com/ETHICON-SURGICEL-SNoW-Absorbable-Hemostat-2082-p/et2082.htm
https://medsurgi.com/surgicel-2082-snow-hemostat-2-x-4/
Additional information
| Amount | Box of 10 |
|---|






Reviews
There are no reviews yet.